


果膠安定劑系列
果膠(英語:pectin),是一類天然高分子化合物,它主要存在於所有的高等植物中,是植物細胞間質的重要成分。果膠沉積於初生細胞壁和細胞間層,在初生壁中與不同含量的纖維素、半纖維素、木質素的微纖絲以及某些伸展蛋白(extensin)相互交聯,使各種細胞組織結構堅硬,表現出固有的形態,為內部細胞的支撐物質。它於1825年被亨利·布拉科諾(Henri Braconnot)第一次分離和描述。
果膠(Pectin)是一組聚半乳糖醛酸。在適宜條件下其溶液能形成凝膠和部分發生甲氧基化(甲酯化,也就是形成甲醇酯),其主要成分是部分甲酯化的α—1,4一D一聚半乳糖醛酸。殘留的羧基單元以游離酸的形式存在或形成銨、鉀鈉和鈣等鹽。
性狀
果膠為白色或帶黃色或淺灰色、淺棕色的粗粉至細粉,幾無臭,口感黏滑。溶于20倍水,形成乳白色粘稠狀膠態溶液,呈弱酸性。耐熱性強,幾乎不溶於乙醇及其他有機溶劑。用乙醇、甘油、砂糖糖漿濕潤,或與3倍以上的砂糖混合可提高溶解性。在酸性溶液中比在鹼性溶液中穩定。
凝膠作用
果膠能形成具有彈性的凝膠,不同酯化度的果膠形成凝膠的機制是有差別的,高甲氧基果膠必須在低pH值和高糖濃度中才能形成凝膠,一般要求果膠含量<1%、蔗糖濃度58%~75%、pH2.8~3.5。因為在pH2.0~3.5時可阻止羧基離解,使高度水合作用和帶電的羧基轉變為不帶電荷的分子,從而使分子間的斥力減小,分子的水合作用降低,結果有利於分子間的結合和三維網路結構的形成。蔗糖濃度達到58%~75%後,由於糖爭奪水分子,致使中性果膠分子溶劑化程度大大降低,有利於形成分子氫鍵和凝膠。
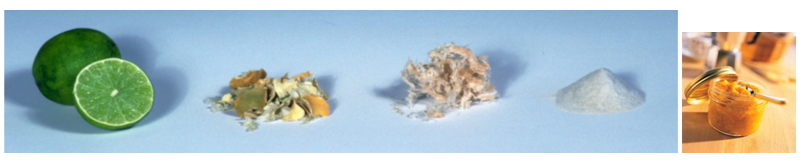
來源
果膠物質是植物細胞壁成分之一,存在於相鄰細胞壁間的胞間層中,起著將細胞粘在一起的作用。不同的蔬菜,水果口感有區別,主要是由它們含有的果膠含量以及果膠分子的差異決定的。柑橘、檸檬、柚子等果皮中約含30%果膠,是果膠的最豐富來源。按果膠的組成可有同質多糖和雜多糖兩種類型:同質多糖型果膠如D-半乳聚糖、L-阿拉伯聚糖和D-半乳糖醛酸聚糖等;雜多糖果膠最常見,是由半乳糖醛酸聚糖、半乳聚糖和阿拉伯聚糖以不同比例組成,通常稱為果膠酸。不同來源的果膠,其比例也各有差異。部分甲酯化的果膠酸稱為果膠酯酸。天然果膠中約20%~60%的羧基被酯化,分子量為2萬~4萬。果膠的粗品為略帶黃色的白色粉狀物,溶于20份水中,形成粘稠的無味溶液,帶負電 。果膠廣泛用於食品工業,適量的果膠能使霜淇淋、果醬和果汁凝膠化。
果膠是一種天然高分子化合物,具有良好的膠凝化和乳化穩定作用,已廣泛用於食品、醫藥、日化及紡織行業。柚果皮富含果膠,其含量達6%左右,是制取果膠的理想原料。果膠分果膠液、果膠粉和低甲氧基果膠三種,其中尤以果膠粉的應用最為普遍。從柚皮中可以制取果膠粉和低甲氧基果膠。
日常生活中,果膠通常從柑橘的果皮萃取,通常呈黃色或白色的粉末狀,具有凝膠、增稠及乳化等作用。果膠也是一種天然的食物添加劑,為製造果醬、果凍、優酪乳及雪糕等。此外,果膠也可為水果保鮮之用。
果膠安定劑系列

Pectin Stabilizer Range
Pectin (English: pectin) is a kind of natural polymer compound, which is mainly found in all higher plants and is an important component of plant cell interstitial. Pectin is deposited in the primary cell wall and intercellular layer, and cross-links with different levels of cellulose, hemicellulose, lignin microfibrils and some extensins in the primary wall, making various cellular tissues hard. It exhibits an intrinsic morphology and is a supporting substance for internal cells. It was first separated and described by Henri Braconnot in 1825 [1] [2].
Pectin is a group of polygalacturonic acids. Under suitable conditions, the solution can form a gel and partially methoxylate (methyl esterification, ie, formation of methanol ester), the main component of which is partially methylated α-1,4-D-polygalactitol acid. The residual carboxyl unit is present as a free acid or forms salts such as ammonium, potassium sodium and calcium.
Traits
Pectin is white or yellow or light gray, light brown coarse powder to fine powder, a few odorless, sticky and smooth. Dissolved in 20 times water to form a milky white viscous colloidal solution, which is weakly acidic. Strong heat resistance, almost insoluble in ethanol and other organic solvents. Wet with ethanol, glycerin, sugar syrup, or mixed with 3 times more sugar to improve solubility. It is stable in an acidic solution than in an alkaline solution.
Gelation
Pectin can form a gel with elasticity. The mechanism of gelation of pectin with different degrees of esterification is different. High methoxyl pectin must form gel at low pH and high sugar concentration. General requirements Pectin content <1%, sucrose concentration 58%~75%, pH 2.8~3.5. Because the carboxyl group dissociates at pH 2.0~3.5, the highly hydration and charged carboxyl groups are converted into uncharged molecules, so that the repulsion between molecules is reduced, the hydration of the molecules is reduced, and the result is beneficial to the molecules. The combination of the three-dimensional network structure. After the sucrose concentration reaches 58%~75%, the solvation of neutral pectin molecules is greatly reduced due to the competition of sugar molecules, which is beneficial to the formation of molecular hydrogen bonds and gels.
source
Pectin is one of the plant cell wall components and exists in the intercellular layer between adjacent cell walls, acting to bind cells together. Different vegetables and fruits have different tastes, which are mainly determined by the pectin content they contain and the difference in pectin molecules. Citrus, lemon, grapefruit and other peels contain about 30% pectin, which is the most abundant source of pectin. According to the composition of pectin, there may be two types of homopolysaccharide and heteropolysaccharide: homopolysaccharide pectin such as D-galactan, L-arabin and D-galacturonan; Commonly, it is composed of galacturonan, galactan and arabinan in different proportions, commonly referred to as pectic acid. The proportion of pectin from different sources varies. Part of the methylated pectic acid is called pectic acid. About 20% to 60% of the carboxyl groups in the natural pectin are esterified, and the molecular weight is 20,000 to 40,000. The crude pectin is a slightly yellowish white powder dissolved in 20 parts of water to form a viscous, odorless solution with a negative charge. Pectin is widely used in the food industry, and the right amount of pectin gels creams, jams and juices.
Pectin is a natural polymer compound with good gelation and emulsion stability. It has been widely used in food, medicine, daily chemical and textile industries. Pomelo peel is rich in pectin, and its content is about 6%, which is an ideal raw material for making pectin. Pectin is divided into three types: pectin glue, pectin powder and low methoxy pectin. Among them, the application of pectin powder is the most common. Pectin powder and low methoxy pectin can be prepared from pomelo peel.
In daily life, pectin is usually extracted from the citrus peel, usually in the form of a yellow or white powder, which has the functions of gelation, thickening and emulsification. Pectin is also a natural food additive for the manufacture of jams, jellies, yogurt and ice cream. In addition, pectin can also be used for fruit preservation.
Pectin Stabilizer Range
